Opinion Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Enhancing Scientific Rigor in Basic Nanomedicine Research for Improved Clinical Translation
*Corresponding author: Chao Wang, The Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins UniversitySchool of Medicine, 733 N Broadway, Baltimore, Maryland 21205, United States.
Received: January 05, 2024; Published: January 10, 2024
DOI: 10.34297/AJBSR.2024.21.002807
Opinion
Over the past two decades, nanomedicine research has surged dramatically due to its unique advantages [1], as evidenced by a substantial escalation in published papers, rising from a mere 2 in 2000 to approximately 4,000 in recent years (Figure 1). Expanding the search to keywords such as “nanoparticle” and “nanomaterial” reveals tens of thousands of associated studies. This expansive field encompasses a wide array of nanomedicines, including liposomes, polymers, organic and inorganic nanoparticles, metal-organic frameworks, and their composite structures. Applications span diverse domains, encompassing cancer treatment, sterilization, anti-inflammatory solutions, Alzheimer’s disease treatment, imaging modalities, and diagnostic approaches. It’s akin to a multifaceted arena where numerous innovations and applications coexist (Figure 1).
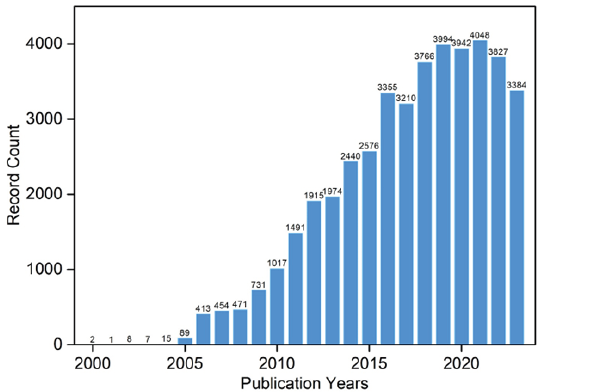
Figure 1: Visualization of the escalating publication counts related to “nanomedicine” over the years, derived from Web of Science data.
However, an unfortunate reality persists: the conspicuously low rates of clinical and industrial translation for these nanomedicines. Presently, only a handful of particle types, predominantly liposomes (primarily employed for drug delivery) [2] and magnetic iron oxide (utilized in MRI imaging) [3], have obtained approval from regulatory authorities such as the FDA for clinical usage. The central query arises: What impedes their translation?
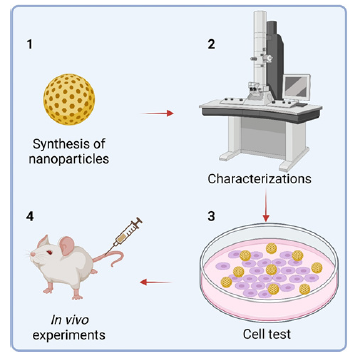
I posit that a critical factor contributing to this plight is the inadequacy of foundational research in nanomedicine. A cursory review of articles on nanomedicine, especially those concerning cancer treatment, usually involves copious experimental data, typically segmented into four primary sections:
1. Nanomedicine preparation.
2. Nanomedicine characterization, encompassing basic and specific profiling.
3. Cell-based tests exploring aspects like endocytosis and toxicity of nanomedicine.
4. In vivo experiments evaluating treatment efficacy, biological toxicity, and tissue analysis (Figure 2).
This trend of numerous data has burgeoned due to ongoing “inflation” within the scientific community over the past decade. Work that once sufficed with cellular experiments a decade ago may now necessitate complete in vivo studies for journal publication at the same level (Figure 2).
While this comprehensive research approach may appear allencompassing, closer scrutiny often exposes inherent loopholes. The complexity of nano therapy, entailing numerous factors (such as particle size, shape, surface charge, density, stability, drug loading capacity, surface modification, unique property testing, binding protein corona, cytotoxicity, haemolysis, endocytosis efficiency, endocytic pathway, intravital injection method, blood circulation half-life, targeting, organ and lesion accumulation, treatment effect, prognosis, short-term in vivo toxicity, long-term in vivo toxicity, etc.) with corresponding multiple parameters mandates meticulous consideration. Even a seemingly straightforward property like particle size demands extensive testing methodologies such as TEM, SEM, and DLS, each with varied parameters and models. For example, in the DLS test, there are intensity, volume, and number models and tens of parameters need to be set up such as temperature, solution, circle time, etc. Researchers must spend substantial time and effort to thoroughly examine a single property. Since they need to complete a series of experimental procedures within a certain period, it is conceivable that the omission of some experiments, even the key experiments, is understandable. And even if all aspects of testing and experiments are finished, it must be superficial and making it difficult to dig into a certain performance.
To address this challenge, there should be some specialized team to study the standard process of a certain test and characterization in the entire process, provide standard results for reference, and thereby formulate industry norms for research. Regrettably, the majority of existing pertinent research papers predominantly adhere to a “standard process” framework (steps 1 to 4 in Figure 2), conducting numerous experiments but lacking in-depth investigations into specific facets of nanomedicine performance. For example, a seemingly straightforward question like identifying the optimal size of nanomedicine for tumor treatment remains elusive due to multifaceted considerations such as the type of nanomedicines, target efficiency, blood circulation time, and lesion accumulation level [4]. Nonetheless, focusing on specific categories or general nanomaterials, such as silica particles, and systematically preparing particles across a range of sizes (e.g., 10nm to 1000nm), followed by in vivo studies in mice, could facilitate comparative analyses. Through discerning the advantages and drawbacks of each size parameter, an approximate optimal size range can be delineated, thereby establishing definitive benchmarks and reference outcomes. Such comprehensive research for a single property of nanomedicine endeavours is poised to lay robust foundations for future studies, alleviating the need for researchers to extensively deliberate over suitable sizes for medical-grade silica synthesis, allowing them to readily adopt established templates.
In a similar vein, dedicated research teams dedicated to exploring individual nanomedicine performance facets aim to develop gold standards or relative reference benchmarks. This concerted effort intends to streamline the application of predecessor results and specifications, expediting assessments of various nanometre scales. This strategic approach not only significantly saves time but also mitigates the squandering of scientific research funds. Two salient aspects warrant consideration in this process. Firstly, referencing the testing and characterization protocols and experimental parameters and procedures employed for widely used clinical or market drugs enables the analogous evaluation of nanomedicines. Secondly, seeking validation from independent and specialized research groups, involving a minimum of 3 to 5 such entities, ensures the verification of repeatability. Achieving an error margin of less than 5% signifies the establishment of a “gold standard” for foundational research or protocols. Ideally, this pivotal step is best executed when journal editors seek reviewers. Notably, high-tier journals, particularly those focusing on protocol dissemination, can request reproducibility verification from renowned and authoritative laboratories upon receiving manuscripts. Once the prescribed standards are met, these protocols attain the coveted label of “top protocols,” serving as a valuable reference for researchers.
Undoubtedly, this issue entails intricate complexities and necessitates multifaceted collaboration, entailing equitable profit distribution among researchers, associations, journals, and pharmaceutical entities. However, considering the colossal resource wastage prevalent in contemporary nanomedicinerelated endeavours-marked by an inundation of meaningless studies and an abysmally low clinical conversion rate-embracing these measures presents an indispensable endeavour.
Acknowledgement
None.
Conflict of Interest
None.
References
- C Wang, S Zhang (2023) Advantages of Nanomedicine in Cancer Therapy: A Review. ACS Applied Nano Materials 6: 22594-22610.
- DJA Crommelin, P van Hoogevest, G Storm (2020) The role of liposomes in clinical nanomedicine development. What now? Now what? J Control Release 318: 256-263.
- RC Popescu, E Andronescu, AM Grumezescu (2014) In vivo evaluation of Fe₃O₄ nanoparticles. Rom J Morphol Embryol 55(3 Suppl): 1013-1018.
- LE Euliss, JA DuPont, S Gratton, J DeSimone (2006) Imparting size, shape, and composition control of materials for nanomedicine. Chemical Society Reviews 2006, 35(11): 1095-1104.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.